Normality Calculator ( Making a solution of solid solute)

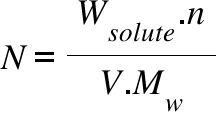
N=Normality of the Solution ; Wsolute = Weight of the Solute ; V = Volume of the solution;
Mw = Molecular Weight of the Solute; n = Equivalence Factor (basicity of an acid or acidity of a base/Number of electron transfer per mole reaction)
You may use the (), however do not proceed the formula with a number e.g. 3H2O use (H2O)3
Normality of a solution is defined as the number of gram equivalent of a solute present per liter of a solution. The gram equivalent is the ratio of molecular weight and equivalence factor. This equivalence factor can be thought as the number of replaceable H+ ions in a molecule of an acidic solute or the number of replaceable OH- ions present in a molecule of a basic solute. Thus equivalence factor of NaOH is 1 and 2 for H2SO4. In the case of redox reactions this equivalence factor is the number of electron taken or given out of that compound in a redox reaction.