Arrhenius Equation Calculator

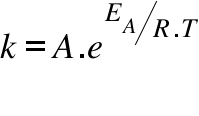
K = Rate Constant; A = Frequency Factor; EA = Activation Energy; T = Temperature; R = Universal Gas Constant ;
Temperature has a profound influence on the rate of a reaction. Arrhenius showed that the rate constant (velocity constant) of a reaction increases exponentially with an increase in temperature. Here 'A' is called the 'pre-exponent factor' or the 'frequency factor' and EA is the activation energy of the chemical process (reaction).