Graham's Law Calculator

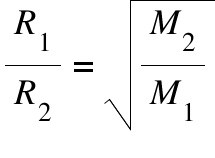
R1 = Rate of effusion (or diffusion ) of the first gas ; R2 = Rate of effusion (or diffusion) of the second gas; M1 = Mass of the first gas; M2= Mass of the second gas
Graham's law states that the rate of effusion (or of diffusion ) of a gas is inversely proportional to the square root of it's molecular weight. The rate of diffusion is the measure of rate at which two gases mix, and the rate of effusion is the measure of rate at which a gas escapes through a pinhole into a vacuum. So, the unit of rate solely depends on the property chosen.