Molarity Calculator (Making a solution of solid solute)

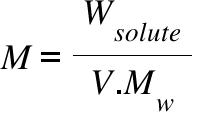
M=Molarity of the Solution ; Wsolute = Weight of the Solute ; V = Volume of the solution; Mw = Molecular Weight of the solute;
You may use the (), however do not proceed the formula with a number e.g. 3H2O use (H2O)3
Molarity of a solution is defined as the number of moles of a solute present per liter of a solution. Thus the molecular weight, volume of the solution , grams of solute present in the solution is related by the formula written above. Molarity of a solution can be calculated using this calculator.