Rydberg Equation Calculator

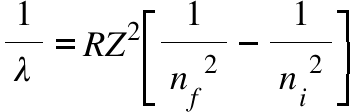
λ= Wavelength of the emmited light (electromagnetic rediation) in the vacuum ; R = Rydberg Constant (1.097x 107 m-1) ; Z = Number of proton in the nucleus of the element; nf = Principal quantum number of final state; ni = Principal quantum number of the initial state
Rydberg formula predicts the wavelength of light (in the vacuum) that is emitted during a electronic transition between different energy levels for Hydrogen, or Hydrogen like elements, such as He+, Li2+, Be3+ etc.