Degree of Dissociation & pKa of Weak Acid

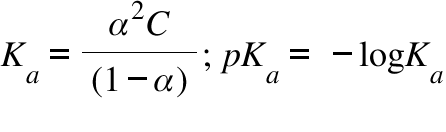
Ka = Acid Dissociation Constant ; C = Concentration of the Acidic Soluiton ;
α = Degree of dissociation of the acid
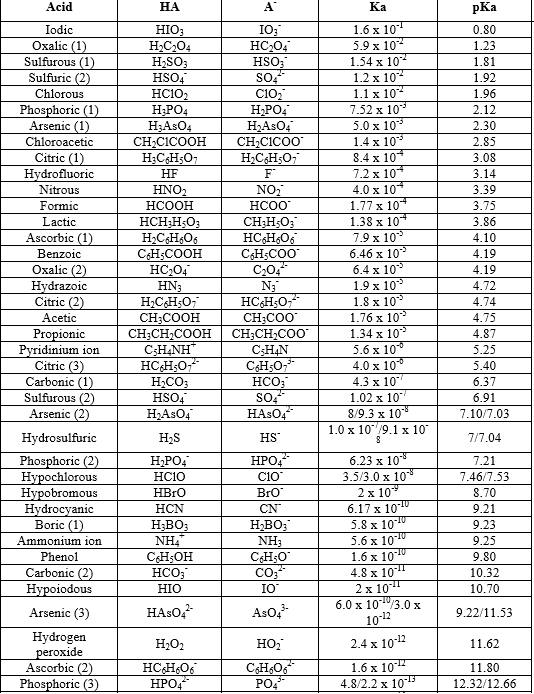
The ionization of an acid in water measures the relative strength of the acid. The Ka is simply the equilibrium constant for the ionization of an acid HA into H+ and A- . It can be written that Ka [H+][A- ]/[HA]. Considering the degree of dissociation to be α we can easily establish the formula involving α , C (=concentration of the solution) and Ka , which is written above. It can be inferred that a higher value of Ka resemble stronger acid. Thus a lower value of pKa which -logKa will resemble a stronger acid. Here is a table of pKa Values: