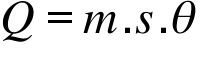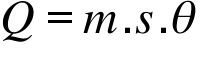
Q= Amount of Heat ; m= mass ; s = Specific Heat ; θ = Temperature Change (a.k.a ΔT)
arkajitmandal,
03.04.2015
|
Posted in

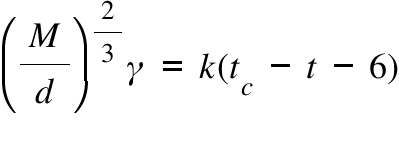
M=Molecular Weight; d= density; γ= Surface Tension ; k=Eotvos-Ramsay Coefficient ; tc= Critical Temperature ; t= System Temperature
arkajitmandal,
01.04.2015
|
Posted in

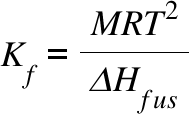
Kf = Freezing Point Depression Coefficient ; M = Molecular Weight of the pure solvent ; R = Gas Constant ; T= Freezing-Point of the pure solvent ; ΔHfus = Heat of Fusion ;
arkajitmandal,
31.03.2015
|
Posted in

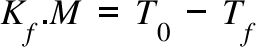
Kf = Freezing Point Depression Coefficient ; M = Molarity of the solution (when solute is added) ; T0 = Freezing Point of the pure solvent; Tf = Freezing Point of the solution
arkajitmandal,
31.03.2015
|
Posted in

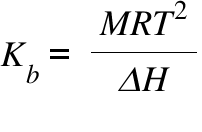
Kb = Boiling Point Elavation Coefficient ; M = Molecular weight ; T= Boiling Point of the pure Solvent ; ΔH = Heat of Vaporization ;
arkajitmandal,
31.03.2015
|
Posted in

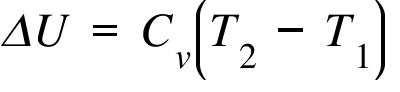
ΔU= Internal Energy Change ; Cv = Heat Capacity at constant Volume ; T2 , T1 = Final and Initial Temperature;
arkajitmandal,
24.03.2015
|
Posted in
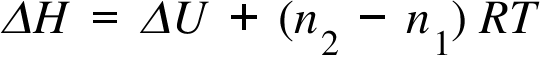
ΔH= Enthalpy change ; ΔU = Internal Energy Change ; n1 , n2 = Gaseous reactant's and Product's Total Mole Number;
arkajitmandal,
23.03.2015
|
Posted in

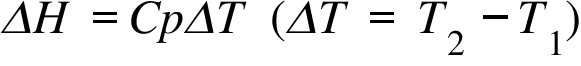
ΔH= Enthalpy Change ; Cp = Heat Capacity at constant Pressure; T1 , T2 = Initial & Final Temperature ;
arkajitmandal,
23.03.2015
|
Posted in

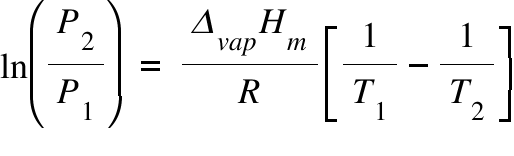
P1 & P2 = Initial & Final Pressure ; ΔvapHm = Molar Enthalpy of vaporization ; T1 , T2 = Initial & Final Temperature ; R= Gas Constant ;
arkajitmandal,
23.03.2015
|
Posted in

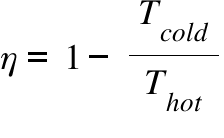
η = Efficiency ; Thot =Temperature of the Hot source ; Tcold = Temperature of the Cold Sink ;
arkajitmandal,
17.03.2015
|
Posted in
Pages