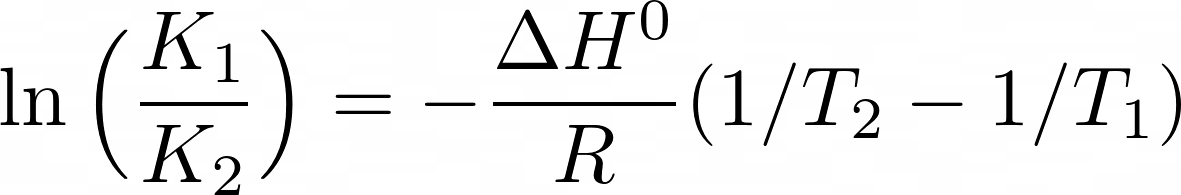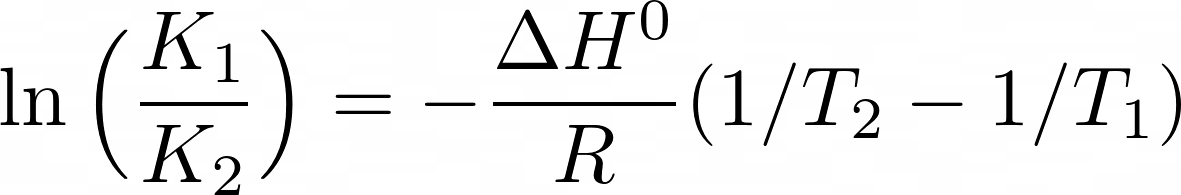
K1= The equilibrium constant at absolute temperature T1 ; K2=The equilibrium constant at absolute temperature T2 ;
ΔHo= The standard enthalpy change ; T1 & T2 = Temperature; R= Gas constant
arkajitmandal,
16.10.2015
|
Posted in

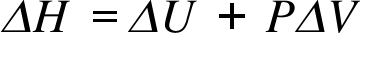
ΔH= Enthalpy Change ; ΔU = (Ufinal - Uinitial) Internal Energy Change; ΔV = (Vfinal - Vinitial)Volume Change;
arkajitmandal,
10.09.2015
|
Posted in

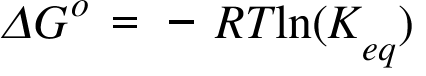
ΔGo= Standard Free Energy Change ; R = Universal Gas Constant; Keq = Equilibrium Constant; T= Temperature
arkajitmandal,
03.09.2015
|
Posted in

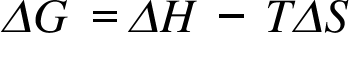
ΔG= Change in Gibb's Free Energy ; ΔH = Change in enthalpy; ΔS = Change in Entropy; T= Temperature
arkajitmandal,
03.09.2015
|
Posted in

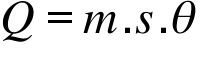
Q= Amount of Heat ; m= mass ; s = Specific Heat ; θ = Temperature Change (a.k.a ΔT)
arkajitmandal,
03.04.2015
|
Posted in

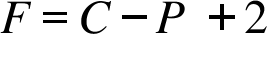
F= Number of Variables ; C= Number of Components ; P= Number of Phases;
arkajitmandal,
01.04.2015
|
Posted in

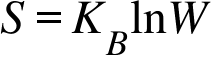
S=Entropy ; KB = Bolzmann's Constant ; W = Number of microsates
arkajitmandal,
25.03.2015
|
Posted in

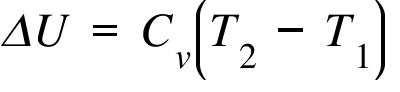
ΔU= Internal Energy Change ; Cv = Heat Capacity at constant Volume ; T2 , T1 = Final and Initial Temperature;
arkajitmandal,
24.03.2015
|
Posted in
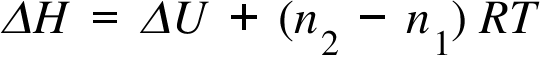
ΔH= Enthalpy change ; ΔU = Internal Energy Change ; n1 , n2 = Gaseous reactant's and Product's Total Mole Number;
arkajitmandal,
23.03.2015
|
Posted in

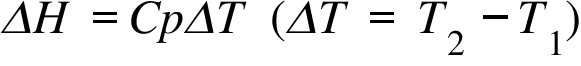
ΔH= Enthalpy Change ; Cp = Heat Capacity at constant Pressure; T1 , T2 = Initial & Final Temperature ;
arkajitmandal,
23.03.2015
|
Posted in
Pages