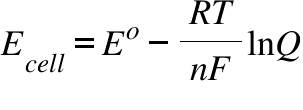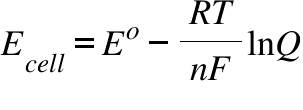
Ecell = Electromotive force of the cell ; Eo= Standard Electrode potential of the cell ; T = Temperature ; Q= Reaction Quotient; n = The number of electrons transferred per cell reaction; R = Gas constant ; F= Faraday's Constant
arkajitmandal,
01.04.2015
|
Posted in

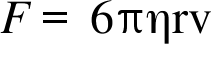
F= Drag Force ; η = Viscosity Coefficient ; r= Radius of the Particle ; v = Relative velocity of the Particle ;
arkajitmandal,
01.04.2015
|
Posted in

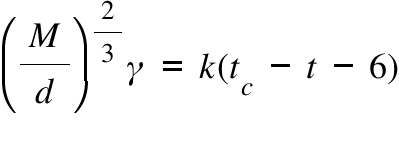
M=Molecular Weight; d= density; γ= Surface Tension ; k=Eotvos-Ramsay Coefficient ; tc= Critical Temperature ; t= System Temperature
arkajitmandal,
01.04.2015
|
Posted in

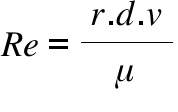
Re = Reynolds Number ; r = The diameter or length (basically length of the shape of the object);
d = The density of the fluid; v = The velocity of the object; μ = Viscosity of the fluid;
arkajitmandal,
01.04.2015
|
Posted in

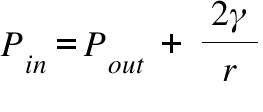
Pin = Pressure Inside the Curved Surface ; Pout = Pressure Outside the Curved Surface ; γ = Surface Tension ; r = Radious of Curvature of the Curved Surface ;
arkajitmandal,
01.04.2015
|
Posted in

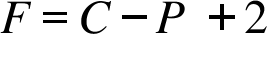
F= Number of Variables ; C= Number of Components ; P= Number of Phases;
arkajitmandal,
01.04.2015
|
Posted in

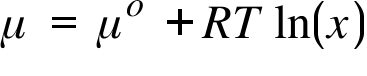
μ = Chemical Potential of a Solution ; μo= Chemical Potential of the pure solvent ; T = Temperature ; R= Gas Constant ; x= Mole Fraction of the Solvent ;
arkajitmandal,
01.04.2015
|
Posted in

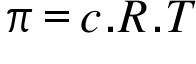
π= Osmotic Pressure ; c= Concentration ; T = Temperature
arkajitmandal,
31.03.2015
|
Posted in

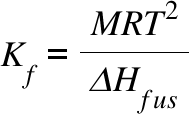
Kf = Freezing Point Depression Coefficient ; M = Molecular Weight of the pure solvent ; R = Gas Constant ; T= Freezing-Point of the pure solvent ; ΔHfus = Heat of Fusion ;
arkajitmandal,
31.03.2015
|
Posted in

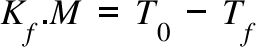
Kf = Freezing Point Depression Coefficient ; M = Molarity of the solution (when solute is added) ; T0 = Freezing Point of the pure solvent; Tf = Freezing Point of the solution
arkajitmandal,
31.03.2015
|
Posted in
Pages