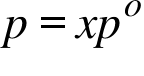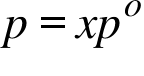
p= Vapour Pressure of an Ideal Solution ; x= Mole Fraction of Solvent ; po =Vapour Pressure of the Pure Solvent ;
arkajitmandal,
31.03.2015
|
Posted in

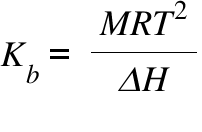
Kb = Boiling Point Elavation Coefficient ; M = Molecular weight ; T= Boiling Point of the pure Solvent ; ΔH = Heat of Vaporization ;
arkajitmandal,
31.03.2015
|
Posted in

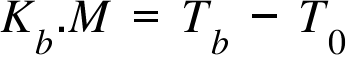
Kb = Boiling Point Elevation Coefficient ; M = Molarity of the solution (when solute is added) ;
T0 = Boiling Point of the pure solvent; Tf = Boiling Point of the solution
arkajitmandal,
31.03.2015
|
Posted in
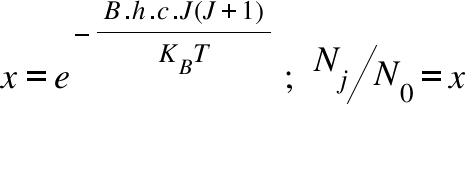
NJ = Number of molecule in J state ; N0 = Number of molecule in the ground state ( J= 0 ) ; KB =Bolzmann Constant ; T = Temperature ; B = Rotational Constant; h = Plank Constant ; x=Relative Population; c = Velocity of light ; J = Rotational Quantum Number
arkajitmandal,
30.03.2015
|
Posted in

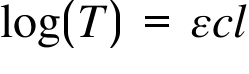
T= Transmittance ; ε = Molar Absorption Coefficient ; c = Concentration ; l = Path Length
arkajitmandal,
30.03.2015
|
Posted in

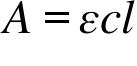
A = Absorbance ; ε = Molar absorption coefficient , Molar absorptivity or Molar extinction coefficient ; c = Concentration ; l = Path Length
arkajitmandal,
26.03.2015
|
Posted in

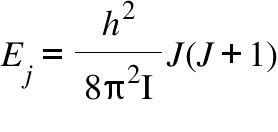
Ej = Rotational energy Level; I= Moment of Inertia; J= Rotational quantum number ; h = Plank's constant
arkajitmandal,
26.03.2015
|
Posted in

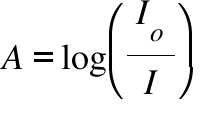
A = Absorbance ; Io = Intensity of the incident radiation ; I = Intensity of the transmitted radiation
arkajitmandal,
26.03.2015
|
Posted in

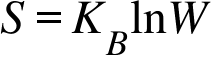
S=Entropy ; KB = Bolzmann's Constant ; W = Number of microsates
arkajitmandal,
25.03.2015
|
Posted in

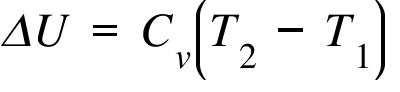
ΔU= Internal Energy Change ; Cv = Heat Capacity at constant Volume ; T2 , T1 = Final and Initial Temperature;
arkajitmandal,
24.03.2015
|
Posted in
Pages